Dear Steve Kirsch: You Owe Me $25,000
The brash millionaire who pimps toxic fluvoxamine as a 'COVID' cure just lost a bet he was probably hoping everyone had forgotten about.
Last week I wrote about millionaire Steve Kirsch, the vaxxxine critic who is vocally pimping a toxic SSRI antidepressant as a 'COVID' cure.
A few days ago, I left a comment underneath his terribly misleading article about the fatally flawed fluvoxamine study from Thailand:
Hi Steve,
you've done a lot of good work in calling out the toxic COVID 'vaccines', so it's terribly sad to see you glorifying a truly garbage drug like fluvoxamine.
Your article ignores several key points:
1. The Thai study you cite in this article is not worth the paper it's written on. It's a completely unblinded, open-label study, meaning the researchers knew which group each subject was in. When one of those researchers, Angela M Reiersen, just happens to hold a patent for the use of fluvoxamine as a COVID treatment, that's a problem. A big problem. The potential for bias and misreporting of the results is obvious.
2. There clearly was misreporting in this study, because the withdrawal data stink like rotting fish on a 40C day. A la the Pfizer vaxxxine study, the treatment groups had a remarkably higher rate of 'withdrawals' than the standard care group, and the researchers don't explain why. This was a randomized trial, and the standard care group was included in the random allocation of subjects, so there should be similar withdrawal rates between groups. Looks to me like the researchers created more 'withdrawals' in the treatment groups to remove subjects with unfavourable data.
Also, zero clinical deterioration and hospitalizations in the combo groups, a mere 9 among the fluvoxamine-only group, yet a whopping 321 of 336 standard care subjects with "mild" 'COVID' experienced clinical deterioration, with many requiring hospitalization?
Yeah, sure.
If this slop was a COVID vaxxxine study, everyone here would be hurling rotten tomatoes at it. Because it's not a vaxxxine, we're supposed to pretend that it's unfairly suppressed research. Sorry, but unblinding, blatant conflicts of interest and extremely suspicious withdrawal and outcome data are not okay just because we're dealing with a non-vaxxxine.
I dismantle this farce of a trial here:
https://anthonycolpo.substack.com/p/dear-steve-kirsch-fluvoxamine-is
3. The gold standard for drug testing is randomized, double-blind, placebo-controlled trials. Something you don't mention here is that there have already been 5 double-blind trials and 1 single-blind trial of fluvoxamine against 'COVID.' Each and every one showed fluvoxamine to be a dud in the treatment of 'COVID.'
Three of those previous trials were conducted by Reiersen and fellow patent holder Eric Lenze, who has financial ties to Jazz Pharmaceuticals, which now holds the license for fluvoxamine.
In one of their trials, STOP COVID 2, fluvoxamine was such a flop that they didn't even bother publishing the results. The other two failed to show any benefit, so the authors chopped, changed and deleted original endpoints in an attempt to contrive something resembling a significant result. They then tried to word their papers as if fluvoxamine was effective. It wasn't, as I explain here:
https://anthonycolpo.substack.com/p/fluvoxamine-a-toxic-and-potentially
Note that the other research groups around the world, who did not have a vested interest in fluvoxamine as a COVID treatment, did not attempt to re-frame their negative results as positive.
Bottom line is that the 3 of 7 trials claiming efficacy for fluvoxamine against COVID just happen to feature Reiersen on the author list, who holds a patent for the use of fluvoxamine against COVID.
4. Fluvoxamine is an SSRI antidepressant. This class of drugs is problematic at the best of times, and fluvoxamine stands out as an especially disagreeable drug. Someone else here wrote that it has a 'superior side effect profile'. Nothing could be further from the truth. It is notorious for causing nausea, agitation, psychiatric disturbances and suicidal behaviour.
The heightened risk of suicidality caused by fluvoxamine is a function of its penchant for causing stimulation and agitation. Depressed people often ideate about suicide, but thankfully most never act upon these thoughts. However, when someone is both depressed and agitated, you have a particularly dangerous state where they are more likely to act upon violent impulses and cause harm to themselves - and others.
The treatment period in the Thai study was 14 days. The peak suicide danger periods with SSRI use occur within the first 4 weeks of treatment, the first 4 weeks after cessation, and after a dosage change.
I've seen with my own eyes what this fluvoxamine junk can do to people, which prompted a 3-part deep dive that you can read at the following links (with plenty of links to the cited studies):
https://anthonycolpo.com/the-great-ssri-scam-how-taking-anti-depressant-drugs-can-kill-you-part-1/
https://anthonycolpo.substack.com/p/the-great-ssri-scam-how-taking-anti-depressant-drugs-can-kill-you-part-2 (this installment contains the most research on fluvoxamine)
I strongly urge you to read my critiques and the research I have linked to, and reconsider your stance on fluvoxamine. It is an awful drug that should have been pulled a long time ago.
Oh, and one last tidbit. You seem to believe the FDA is 'suppressing' fluvoxamine. Here's the reality: Fluvoxamine's US approval in the US was achieved on the basis of a fraudulent application by then-owner Solvay:
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/021519s000_MedR.pdf
I suspect this came to light because of the controversy caused when it was revealed Columbine shooter Eric Harris had been taking fluvoxamine at the time of the shootings.
Fluvoxamine was temporarily withdrawn from the US market in 2002. Despite Solvay's egregious dishonesty, and nothing having materially changed about fluvoxamine itself, the drug was quietly green-lighted again by the FDA in 2007.
Perhaps to assist this ruse, Solvay had licensed the right to market Luvox to Jazz Pharmaceuticals, pending FDA approval to reinstate the drug for sale in the US. While all this was going on, fluvoxamine remained available in the US in generic form.
Does that sound to you like a drug the FDA is trying to suppress?
The reason fluvoxamine has not been approved as a treatment for 'COVID' is simple: Repeated double-blind studies have shown it is useless as a treatment for 'COVID.' The data is so poor that there's no amount of statistical sorcery that can hide the fact this drug is useless for that purpose.
There are so many better options for dealing with respiratory and flu-like ailments that I can't fathom why people look to toxic drugs like fluvoxamine.
Kind regards,
Anthony Colpo.
Three days later, and that comment remains ignored by Kirsch.
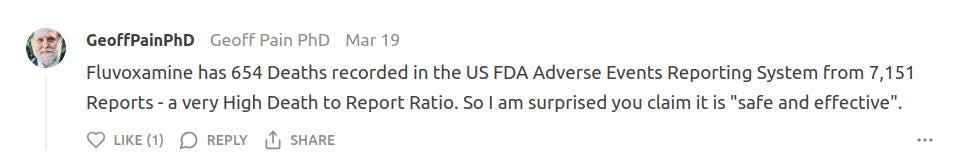
As has a comment posted on March 19 by Geoff Pain, PhD, pointing out that fluvoxamine has 654 deaths recorded in the US FDA Adverse Events Reporting System from 7,151 reports - a rather high death to report ratio. Kirsch loves citing the VAERS database when highlighting the harms of the 'COVID' gene therapies, but seems uninterested in the FDA database entry for fluvoxamine.
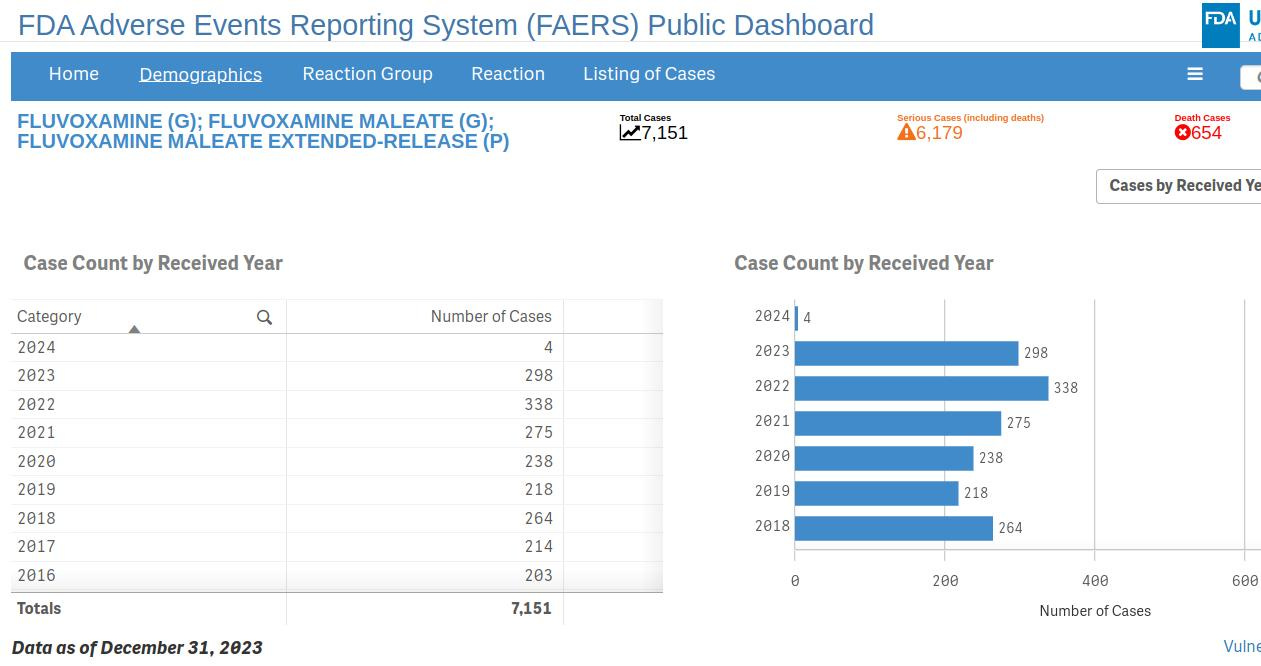
I just headed on over to the FDA AER dashboard to check the fluvoxamine entries for myself. Geoff is correct - it shows 7,151 AERs for fluvoxamine and its extended release formulation, of which 6,179 have been deemed serious cases. There have indeed been 654 fluvoxamine-related deaths reported to the database.
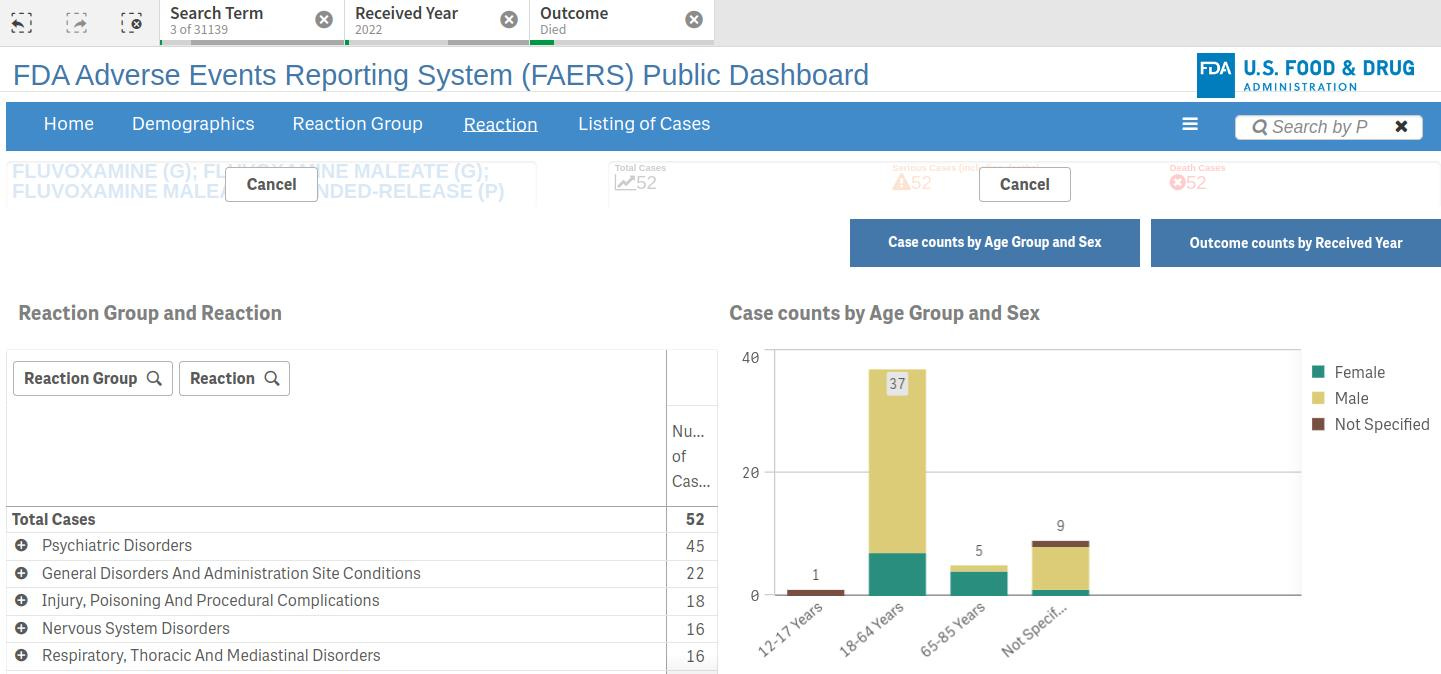
The chronological chart for these reports shows that 218 adverse event reports were for fluvoxamine in 2019, right before the ‘pandemic’ kicked off. By 2022, the number of AERS had risen to 338, including 52 deaths.
Analyses of the VAERS system suggests as little as 1% of adverse vaccine events make it onto the database, and there's little reason to assume the clunky, user-unfriendly FDA database would be any different. Meaning the true number of AERs and deaths due to fluvoxamine is likely much higher.
I can't help but suspect this recent increase in fluvoxamine-related adverse events is due to Kirsch and his aggressive pimping of fluvoxamine as a 'COVID 'cure.'
His recent pro-fluvoxamine article, sadly, shows that many of his followers have bought into his absurd thesis that fluvoxamine is a Safe & Effective™ 'COVID' therapy (gee, where have we heard that before) that is being malevolently suppressed by the FDA in order to protect Big Pharma (who just happens to produce fluvoxamine).
Kirsch's readers have given his appalling article 435 likes as of this writing, and it's clear from the comments section that many are now toying with the idea of adding fluvoxamine to their anti-foo-foo virus arsenal. Like this person:
For heaven's sake ... no!
On Dec 10, 2020, Kirsch posted an article claiming fluvoxamine is "The fast, easy, safe, simple, low cost treatment for COVID that has worked 100% of the time to prevent hospitalization that nobody wants to talk about."
This utter nonsense has been accompanied by a string of interviews and media appearances where he has effusively praised the dangerous antidepressant, which has become the least-prescribed SSRI thanks to its problematic side effect profile.
Oh, and did I mention how in 2020, Kirsch was even pimping remdesivir, affectionately known as rem-death-is-near, as a COVID treatment?
While asking for donations to his now-defunct COVID-19 Early Treatment Fund (CETF), he wrote: "We now know for a fact that remdesivir is a silver bullet against this virus in monkeys if given early enough. And we know remdesivir is safe enough to give to even very sick people."
That didn't age well.
Interestingly, while accusing the FDA of conspiring against fluvoxamine, Kirsch has nothing to say about the FDA granting an EUA to remdesivir despite its poor showing in human trials and its documented kidney-wrecking effects.
When I observed Kirsch's highly selective approach to drug and FDA criticism, and how he blissfully ignored the comments of Geoff Pain and yours truly, I started to smell a rat. Kirsch is a guy who positions himself as a bold warrior of anti-vaxxx truth. He brashly issues challenges to all and sundry, and loves to boast that no-one wants to debate him.
That's simply not true.
Issuing Disingenuous Challenges You Can't Lose - Or Won't Respond To
On June 11, 2023, Kirsch posted a Substack article titled:
"Will any qualified person challenge me 1-on-1 on anything I claim to be true? I doubt it."
"I’m getting the feeling," says Kirsch, "nobody with any academic credentials wants to challenge me on anything I say." (Bold emphasis added).
He then issues a challenge to debate anyone who can prove him wrong on anything he's ever said, in writing or verbally.
Well, not quite anyone, it turns out.
Despite complaining in the very same article about people who use the “you aren’t qualified” excuse to avoid debating him, he proceeds to enact his own highly arbitrary qualifying criteria.
"To accept my challenge," declares Kirsch, "simply reply to the PINNED comment below with these 6 items:
Name: Your real name
Content: Your contact info (e.g., if you use your Twitter account, give me your twitter hand and follow me so I can DM you)
Followers: Evidence that you have at least 1,000 followers on Twitter or other social media (i.e., that people respect you), e.g., a link to your profile page
Bio: A link to your bio
H-index: Proof you have an h-index >= 7 (mine is 7).
Topic: The specific topic you want to challenge me on, e.g., vaccines cause autism, vaccines in general are bad, etc."
On March 19, I replied to Kirsch's pinned comment with all the above information, as reprinted below:
My name is Anthony Colpo. I am approaching 3,000 subscribers/followers on Substack.
I have one peer-reviewed, English-language paper to my name on a biochemical/physiological health topic (the same number as you before your recent paper was questionably retracted), on the subject of cholesterol:
https://www.jpands.org/vol10no3/colpo.pdf
According to Google scholar, that one article has been cited by other authors 176 times - almost triple the number of your most widely-cited paper, yet my H-index is 3 (it was actually well over 7 before I removed all my non-peer reviewed web articles):
https://scholar.google.com/citations?hl=en&user=Iow9P2oAAAAJ
If we only considered published papers pertaining to biochemistry/physiology topics, you too would fail to meet your own arbitrary H-index requirement.
My bio: I'm an autodidact that holds certifications in fitness training. I have no university qualifications (started sports science and law courses around a decade ago but dropped out in disgust - I don't make a good robot), but have easily and routinely outpointed those who do (for an example, see http://www.jpands.org/vol11no1/correspondence.pdf). When I sat the STAT test here in Australia over a decade ago, I was told I did better than 88% of the Australian population. So, even the system can't tell me I'm stupid because, by their own tertiary admission standards, I actually scrub up quite well. I've read literally thousands of journal papers and have published four books.
I agree with you that the vaxxxines are dangerous. I could have told you this well before you listened to all those commentators with the mass appeal you respect and got double Moderna'd.
My issue is with your recent claim that fluvoxamine is an effective 'COVID' treatment.
This is dangerously false, and I explain why in detail at the following links:
https://anthonycolpo.substack.com/p/fluvoxamine-a-toxic-and-potentially
https://anthonycolpo.substack.com/p/dear-steve-kirsch-fluvoxamine-is
I also left the following comment at the bottom of your fluvoxamine article:
I am happy to debate you on this. I'd also be more than happy for you to simply explain in writing why you praised fluvoxamine on the basis of a totally unblinded junk study sporting extremely suspicious withdrawal and clinical data, where one of the researchers has a clear vested financial interest in attaining favourable results?
Why did you gush over this study when previous double-blind and single-blind studies showed fluvoxamine to be a flop in treating 'COVID'?
Why are you giving praise to a problematic SSRI with a track record for causing nausea, agitation and suicidal behaviour?
Why are you praising fluvoxamine and the Thai study after clearly having done so little background research on the drug and the researchers involved?
How did you miss the obvious and glaring anomalies in the study?
Did you read the paper for yourself, or did you rely on the opinion of your scientific friends you "trust," something you often do as you admitted in your article asserting that Sars-Cov-2 has been isolated?
I eagerly await your response. You can DM me through Substack.
Note how Kirsch demands a H-index of 7 or more. This is a ranking that weighs the number of articles you've published against the number of times you've been cited by other researchers.
Kirsch gives no explanation whatsoever why a H-index of 7 is an appropriate threshold. He apparently has chosen it for no other reason than he himself has a H-index of 7.
Kirsch cites his Google Scholar H-index rating, so I created a profile on Google Scholar. After removing everything but my published books and the English and Spanish versions of my 2005 JPANDS paper, my H-index was 3.
Might not sound impressive, but if we took the number of papers Kirsch has published discussing biochemical/physiological topics (not tech-related papers from over 40 years ago), his H-index would be zero.
Here on Substack, there are hordes of very astute and knowledgeable writers with a far better grasp of the current COVID shiteshow than Kirsch, who would nonetheless have a zero H-index ranking because they've never published a peer-reviewed paper.
Meanwhile, there are countless Big Pharma-funded liars who have very impressive-sounding H-index scores. Many of their papers were written by ghost writers - all they did was sign their name to the papers and receive nice fat checks from the companies as a thank you for prostituting themselves. When you're the recipient of lucrative Pharma and government grants, along with drug company perks and sleaze funds, it's quite easy to build up an impressive-looking H-index.
When you're a solo writer eeking out an income by unrepentantly pointing out unpopular facts ... not so much.
So by setting his arbitrary H-index, Kirsch cleverly eliminates the real thinkers and largely confines those who qualify to easily-debunked pharma stooges. The kind of idiots who sign their names to papers they haven't even read, let alone written.
It would be like me loudly issuing a fight challenge to "anyone" ... with "anyone" being only those with one leg and weighing less than 65 kg.
He also stipulates that to qualify, challengers must have a following on social media or Substack of 1,000 or more. While I qualify on that count, I really don't see how one's social media following even begins to qualify as an "academic" credential. There are scores of vacuous, heavily botoxed, fake-tanned bimbos and himbos out there with hundreds of thousands of followers on social media who probably need help tying their shoe laces, yet going by Kirsch's criteria these people are Einsteins in the making! All they need to do is get a ghost writer to boost their H-index to the lucky 7 mark, and Kirsch will debate them ... while ignoring truly intelligent people.
Another person Kirsch has ignored is Rabbi Yitzchok Dovid Smith. Kirsch is a staunch believer in the mainstream 'virus' theory, with its fanciful tales of leaky Chinese biolabs, gain-of-function sorcery, scary new variants and all the other fear-porn bollocks we've been hammered with over the last 4.5 years.
The good Rabbi took issue with an article Kirsch wrote, an article that - whatever Kirsch's real intention - served to further bolster the ‘gain of function’ fear porn campaign.
Rabbi Dovid Smith had already explained why the Chinese biolab theory was nonsense, part of the massive psy-op being conducted upon billions of unsuspecting victims.
As the quote below shows, Rabbi Dovid Smith gets it.
"But our minds and hearts are not only being played with to buy the 'mainstream narrative,'" he writes. "They are also being played with to buy a series of 'alternative' viewpoints. Whichever side you choose, the enemies of liberty score because you have opted to leave common sense and instead choose a worldview that will lead you into surrendering your own liberty."
Bullseye!
The Chinese biolab, man-made virus story was deliberately propagated to convince people that this was a scary new entity, the likes of which the world had never seen before. Which, of course, would require a new treatment the likes of which the world had never seen before.
Enter gene therapies containing Chimpanzee adenoviruses (AstraZeneca) or mRNA technologies with a 30-year track record of failure (Pfizer, Moderna).
After telling Kirsch he would like to debate him, Rabbi Dovid Smith said of the H-index requirement:
"Please consider that the h-index criteria propagates the Cult of Experts, giving value to only one narrow measure, excluding most human beings with something valuable to say. Access to these publications requires participation in the editorial and peer scheme. Some of the authors you champion had their works removed and would have a zero h-index rating. Other authors have high scores and nothing valuable or true to say."
Bullseye again.
The response from Kirsch?
Silence.
Kirsch has also ignored my response to his challenge.
So if you're an easily-debunked, evasive pharma stooge like Paul Offit or Peter Hotez, then yes, Kirsch will probably debate you.
If you can prove Kirsch wrong on something, and you are qualified to do so because you actually know what you're talking about, Kirsch pretends you don't exist. He invents arbitrary criteria based solely on mainstream acceptance and popularity, which automatically removes from contention many of us who are both knowledgeable and the targets of cancel culture.
By setting his arbitrary criteria, Kirsch can use plausible deniability to avoid being proven wrong by someone whose following is only a fraction of his.
Because that would be really embarrassing, wouldn't it?
Steve Kirsch: Pseudoscientist Extraordinaire
As I've detailed here and here, fluvoxamine has repeatedly failed to show efficacy as a 'COVID' treatment in 5 double-blind trials and 1 single-blind trial, conducted by various research groups around the world.
Of course a trivial little thing like repeated failure under double-blind conditions - the gold standard of drug research - isn't going to stop Kirsch.
Instead he defers to utterly uncontrolled slop as 'proof' that fluvoxamine is a "100% effective" 'COVID' treatment.
He gushed like a 60s Beatles fan over the recently-published open-label, totally-unblinded, contactless trial in Thailand.
One of the authors of that totally unblinded trial just happened to be Angela Reierson, she of the vested financial interest.
Which may help explain why there were a strikingly higher number of mysterious researcher-initiated withdrawals in the treatment groups, compared to the standard care group. 'Withdrawing' treatment group subjects with unfavourable results from the study could easily have turned the results around in favour of fluvoxamine.
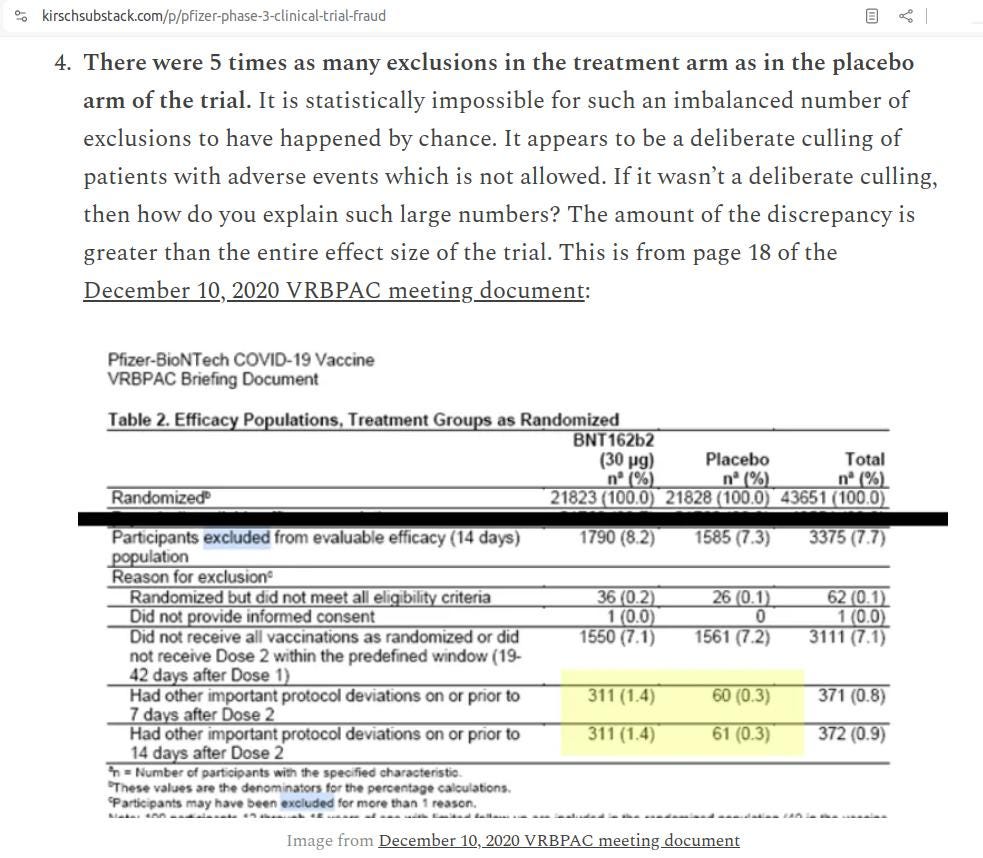
When the Pfizer vaxxx trial contained an inexplicably higher number of withdrawals in the drug group, Kirsch was over it like a rash. Here's an excerpt from an article he wrote about fraud in the Pfizer vaxxx trial, citing the highly suspicious five-fold number of exclusions in the vaxx group when compared to the placebo group:
So Kirsch is rightly suspicious when Pfizer nonchalantly reports excluding 5 times more vaxxx participants, but when a fluvoxamine trial containing an undeniable conflict of interest inexplicably reports 4 times as many withdrawals in the treatment groups, Kirsch completely ignores it.
That’s called hypocrisy.
I've since discovered that Kirsch and Reierson are by no means strangers: Kirsch’s now-defunct COVID-19 Early Treatment Fund (CETF) actually funded Reiersen and her co-author Eric Lenze, both of whom hold a patent for the use of fluvoxamine in treating ‘COVID-19.’ So Kirsch is well aware of the vested financial interest these researchers have in the subject.
And he ignores it.
The Great Racetrack Wank
If there's one thing Kirsch clearly loves, it's poor quality evidence.
He provides easy material for anti-anti-vaxxxers by conducting unscientific surveys on his Substack (eg, "This could be the most important survey you'll ever take"), which he then holds up as proof of vaxxx harms.
Regarding fluvoxamine, he has repeatedly trumpeted an anecdotal tale about the Golden Gate Fields race track as “direct evidence” fluvoxamine is a "100% effective" COVID treatment.
The story goes like this:
In November 2021, the Golden Gate Fields horse racing track in Berkeley, California experienced what Kirsch calls "a massive COVID outbreak." "Massive" being 200 people who tested positive for the never-isolated Sars-Cov-2 according to the inherently-flawed and fraudulent COVID PCR test.
In response to this textbook classic case-count hysteria, Dr. David Seftel, the Harvard/UCSF-trained physician for the track, reached for clinically unproven fluvoxamine. The impetus was the small 2020 study in JAMA by Lenze et al (aka STOP COVID), that implied (but did not demonstrate) efficacy for fluvoxamine. Seftel, says Kirsch, "found the study results impossible to ignore."
Seftel did not find it impossible, however, to ignore the study’s numerous flaws.
He was able to ignore the fact the trial was conducted by Reiersen and fellow patent holder Lenze.
He ignored their absurd claim that a supposedly inert placebo produced more serious adverse gastrointestinal effects than the maximum daily dose (300 mg) of fluvoxamine - a drug notorious for causing nausea!
He ignored the fact that 181 subjects, not 152 as reported in the abstract, had originally been randomized to receive fluvoxamine or placebo, and that 29 subjects were dropped at or prior to baseline, in most instances for reasons that were not fully explained. So again, we see the unexplained 'withdrawals' red flag, which can be used to conveniently omit inconvenient results in the drug group (a la the Pfizer vaxxx trial, which Kirsch is quick to cite).
Seftel ignored the fact that, despite ‘COVID-19’ being sold as the deadliest disease ever, there were no deaths in the study. At least among the subjects that weren't mysteriously withdrawn by the patent-holding researchers.
He ignored the fact that monitoring of moderate-to-serious adverse events was the responsibility of two of the study authors, one of whom just happened to be the antidepressant-friendly and Jazz-remunerated Lenze (Jazz hold the US license for Luvox).
He ignored the fact that, due to reigning COVID hysteria, the trial was conducted in a remote, contactless manner. The participants were assessed during the trial, not by trained medical staff, but by themselves using equipment delivered to their door during quarantine for which they had received no training (this included an oxygen saturation monitor, automated blood pressure monitor, and a thermometer). The study's endpoint data, therefore relied upon patient self-report and self-measurement of symptoms.
Despite this, there was no difference between groups in change of the most severe baseline symptom. Rather strange for a drug he and Kirsch would later claim was "100% effective!"
Seftel ignored the fact that one of the original secondary endpoints was the severity of daily improvement in symptoms as rated by the subjects themselves, but that this endpoint was subsequently abandoned "because the daily trajectory curves of the two groups) curves showed no substantial differences."
Seftel also ignored the fact that the study protocol was quietly amended in April 2020 to remove the original "exclusions for currently taking chloroquine, hydroxychloroquine, azithromycin or colchicine."
So the results may have been confounded by other drugs purported to exert a therapeutic effect of the re-badged cold/flu known as COVID.
Or maybe he didn't ignore them. Maybe, at prestigious robot factories like Harvard and USFC, they simply don't teach you to read study protocols and look for these kinds of discrepancies in study papers.
Kirsch writes that Seftel then offered the drug to "COVID-infected" employees (ie, employees who tested positive via PCR, which original PCR inventor Kary Mullis stressed is not proof of active infection).
"Although everyone at the racetrack adores Dr. Seftel," writes Kirsch, "only 35% of the patients chose to take the drug.”
"Just two weeks later," Kirsch continues, "the acceptance rate is 100%."
Which proves nothing except that it's easy to convince Americans to take drugs.
"Even more impressive," gushes Kirsch, "is that patients who initially refused the drug are now coming back asking for the drug because they saw the results with their own eyes: this is a remarkably tight-knit community and the difference between their friends who took the drug vs. rejected the drug was as clear as night vs. day. Word spread fast. Anyone who took the drug shrugged off the virus like a mild cold."
Which is exactly what most so-called COVID cases were: A mild cold. We knew early on that the infection fatality rate of so-called COVID was the same as seasonal influenza. That's because COVID was seasonal influenza re-packaged as a deadly, novel, jet-setting, cruise-booking virus from China. Do Kirsch and Seftel not wonder why regular influenza magically vanished when COVID appeared on the scene?
Kirsch continues:
"The local Berkeley newspaper observed that the hospitalization rate was so low ('a handful of people') despite the massive number of COVID-postive cases. This was what I like to call the 'Miracle at the Racetrack.' The newspaper wasn’t allowed to contact the employees to find out why. So nobody knew what caused this miracle."
Well, we already know what did not cause this "miracle": Fluvoxamine.
As I've already explained, it has repeatedly failed in blinded trials to exert any beneficial effect on 'COVID'.
So what would explain the alleged 'miracle'? Well, we can't find out from the patients, because Kirsch claims the newspapers were unable to find even a single patient willing to step forward and share their miraculous experience.
I'm not sure if Seftel and Kirsch have ever heard of the "placebo effect," but it's real and it's powerful. That’s why we have blinded, placebo-controlled studies.
Duh.
Here's a hypothetical example of how the placebo effect might work:
Dr S is a doctor in Berkeley, reportedly adored by people who work at the local racetrack. When an outbreak of re-badged flu breaks out at the track, Dr S prescribes the toxic SSRI called fluvoxamine. He does this based on a small study riddled with obvious shortcomings.
At first, most people are reluctant to take an antidepressant to treat a respiratory ailment because, let's face it, that's stupid.
However, 35% of the people adore Dr S so much they go ahead and take the antidepressant. As their colds and flu clear up of their own accord, and unaware that the COVID infection fatality rate is only a fraction of a percent, these patients assume that fluvoxamine has saved them from almost certain death.
"Wow, it's a miracle!" they exclaim.
Excitedly suffering this delusion, and perhaps experiencing fluvoxamine's documented stimulant effects (greatly exacerbated by caffeine), they begin telling everyone who will listen in their tight-knit community about this wonder drug.
Before you know it, 100% of "patients" agree to take the drug, and they too swear that fluvoxamine has saved their lives in the face of a 'pandemic' with a 0.27% infection fatality rate.
"It's uh-maay-zing!" exclaim these 65% of patients who were once smart enough not to take an antidepressant to treat a mild cold, but have now succumbed to the power of placebo and good old-fashioned BS.
Dr S excitedly shares this textbook classic example of placebo effect with millionaire tech entrepreneur Steve K, who has a reputation for shouting at journalists over the phone and sending them emails written almost entirely in ALL-CAPS. Steve K, true to his bombastic nature, proceeds to vocally repeat Dr S's entirely anecdotal tale as 'proof' that fluvoxamine is "100% effective" against COVID.
Yet Another Challenge…
Steve Kirsch was so sure of fluvoxamine's efficacy that on March 21, 2021 he issued the following challenge:
"$25,000 reward if you can show we need more data re: Fluvoxamine for COVID-19"
Kirsch writes:
"Traditionally, a phase 2 trial plus a phase 3 trial, both with statistical significance, is accepted as evidence. That's a p-value of .0025 or lower. NIH Francis Collins said an effect size of 20% or more would be significant. For both published randomized studies for fluvoxamine, the effect size was 100%."
Here's what the FDA, which grants drug approvals in the US, actually says:
"Generally, the agency expects that the drug maker will submit results from two well-designed clinical trials, to be sure that the findings from the first trial are not the result of chance or bias."
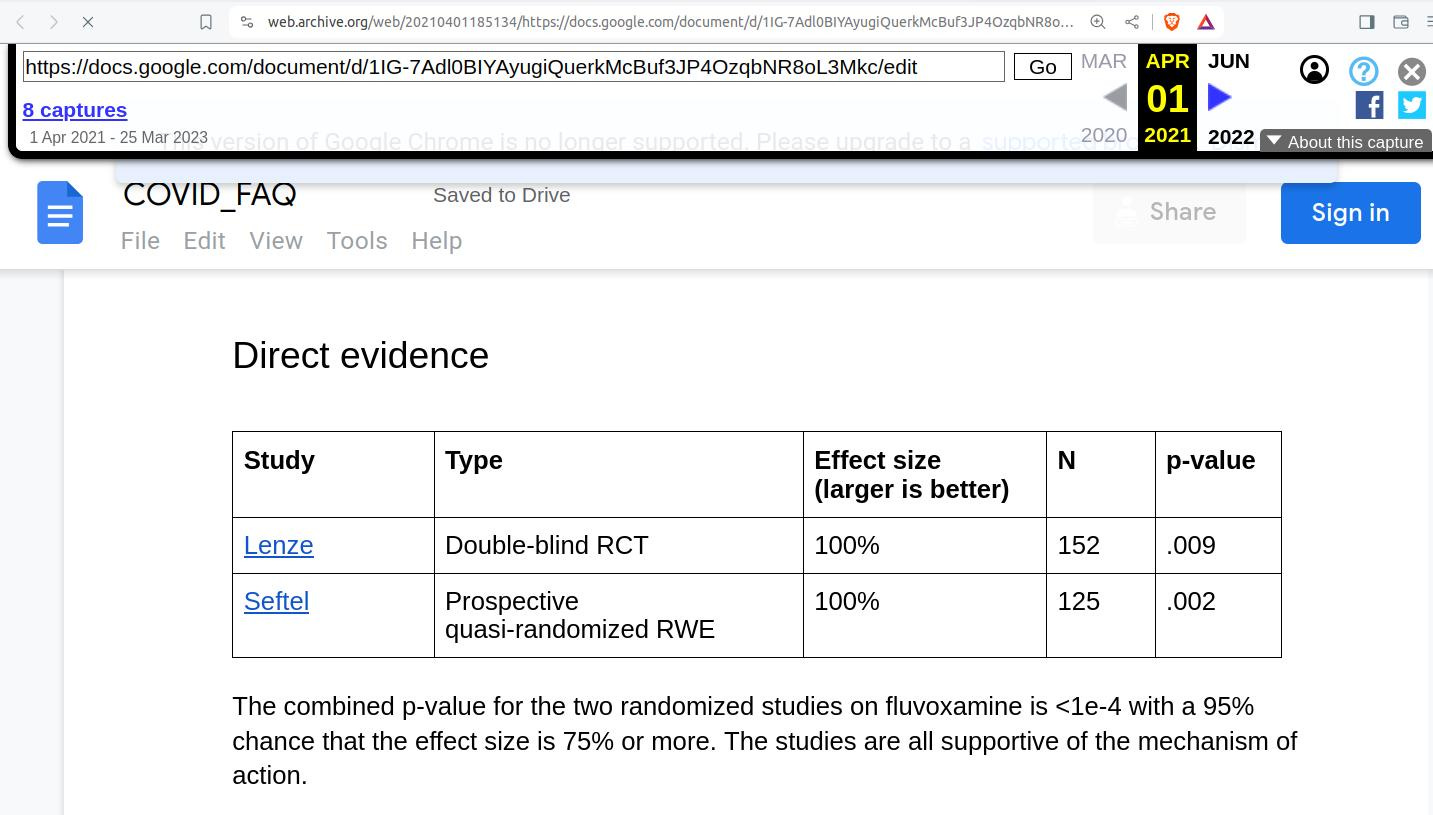
The two studies that Kirsch submits as "direct evidence" that we don't need any more data, and that the FDA should just go ahead, throw caution to the wind, and approve fluvoxamine for 'COVID,' are:
"Lenze", which is actually Lenze et al 2020.
"Seftel", which is actually Seftel and Boulware 2021.
The cavalier Kirsch declares:
"I'm offering a $25,000 to the first person who can provide evidence that shows that the combined evidence from studies published in peer reviewed journals don't already meet that bar.
This means you need to be able to produce actual proof (i.e., hard evidence) that either:
There is a .25% chance or more that these results could be produced by pure chance
that there is a a 95% chance or more that the effect size is less than 20%."
Lenze et al claimed 0% of fluvoxamine and 8.3% (6 subjects) of the placebo group experienced clinical deterioration, with a P-value of 0.009.
It should be noted there were no differences in "Most severe baseline symptom change score" nor "30-d post trial observation events (emergency department visit, hospitalization, or both)."
Whatever the statistical strength of the clinical deterioration finding, this study was riddled with flaws, which I have already noted above.
But don't take my word for it - here are the authors themselves in the mandatory limitations discussion of their paper (bold emphasis added):
"...because of study limitations, these findings need to be interpreted as hypothesis generating rather than as a demonstration of efficacy."
"This study has several limitations," they go on to admit. "First, it was a small study and it was conducted within a single geographic area, so these findings should be regarded as preliminary. The study needs to be replicated in larger trials with a more heterogeneous study population."
“Second, there was a small number of end point events, which makes the findings extremely fragile.”
“Third, it is possible that the differences in clinical deterioration may have been a reflection of the comparative baseline distributions of oxygen saturation rather than an effect of treatment.”
“Fourth, the method of measuring the most severe baseline symptom over time did not appear to provide valid data, so potential effects of fluvoxamine on symptomatic improvement are unknown. “
“Fifth, 20% of study participants stopped responding to surveys during the 15-day trial. Although it was confirmed that none of these participants required medical care, such as hospitalization or an emergency department visit, it is possible that some received care at an urgent care center outside the major regional hospital systems."
“Sixth, the follow-up duration was short and did not measure the effect of fluvoxamine on persistent symptoms or late deterioration.”
“Seventh, the 7-point ordinal scale created for this study to classify clinical deterioration has not been validated.”
In light of these limitations and the study’s highly suspicious adverse event data, it’s hard to imagine a flimsier basis on which to claim efficacy for fluvoxamine. In stark contrast to their abstract, even the authors are admitting their study was flawed and cannot be taken as proof of anything.
As for Seftel and Boulware 2021, not only is it not a "well-designed" clinical trial, as stipulated by the FDA, it's not even a Phase 2 or Phase 3 trial as stipulated by Kirsch. In fact, it's not a clinical trial of any sort. As you might have guessed, the topic of the paper was Seftel's entirely anecdotal and uncontrolled Golden Gate Fields racetrack experience.
Seftel and Boulware (and Kirsch) bizarrely describe their study as "quasi-randomized," but there was no randomization whatsoever; the paper clearly states the choice to take fluvoxamine "was at the patient’s discretion."
Here on Planet Earth, the entire point of randomization is to remove patient discretion from group allocation in a study. This prevents people with characteristics such as greater health consciousness choosing the treatment group, a phenomenon which could easily sway the results of the study in a direction that the actual treatment had nothing to do with.
The bottom line is that Kirsch completely fails to present two randomized clinical trials showing efficacy for fluvoxamine that meet his own stipulated statistical criteria.
He presents one deeply flawed trial in which the authors admit, deep in the paper, that the results cannot be taken as a demonstration of efficacy. The other study he presents is not even a clinical trial but an anecdotal multiple case report of the Golden Gate Fields racetrack story.
So by his own standards, Kirsch has just lost his $25,000 bet.
Before I write to Kirsch and tell him he owes me US $25,000, let's see what other clinical trials on this topic were published by March 21, 2021, when Kirsch was claiming there was sufficient evidence.
The answer is none.
The remaining single-blind and double-blind trials (discussed in detail here), were all published in 2022-2023. And they soundly failed to establish fluvoxamine as an effective treatment for ‘COVID-19.’
In January 2023, JAMA - the same journal that published the original Lenze et al trial in 2020 - featured an editorial by Bhimraj and Gallagher titled "Lack of Benefit of Fluvoxamine for COVID-19."
Reflecting on the failure of the ACTIV-6, STOP COVID 2 and COVID-OUT trials, and considering the doubtful results of the Lenze et al and TOGETHER trials, the authors concluded "the totality of evidence for fluvoxamine does not support its current use for treatment of mild to moderate COVID-19."
The Bottom Line
The bottom line is that on March 21, 2021, Steve Kirsch was not able to present two randomized clinical trials, either Phase 2 or 3, that would meet drug approval requirements nor his own statistical requirements, showing efficacy of fluvoxamine for COVID, be it in the first 7 days of administration or for any other duration.
Phase 2 trials, by the way, are primarily designed to provide safety and not efficacy data, according to the FDA itself.
In contrast, I have presented three double-blind trials (ACTIV-6, STOP COVID 2, COVID-OUT) and one single-blind trial (Seo et al 2022) that failed to show any statistically significant benefit for fluvoxamine in the treatment of ‘COVID.’
I have also explained in detail why Lenze et al (STOP COVID), TOGETHER and the recent completely unblinded and highly suspicious Thai trial do not even begin to constitute valid evidence of efficacy for fluvaoxamine.
I have also pointed out that of seven trials to date, the only three claiming efficacy for fluvoxamine against COVID all happen to feature Angela M Reiersen, who has a clear vested financial interest in attaining positive findings.
I have clearly demonstrated that the weight of the evidence does not support the use of fluvoxamine in treating ‘COVID.’
JAMA, the journal that helped kick off the fluxovamine fascination when it published Lenze et al 2020, has now published an editorial agreeing with me.
Kirsch lost his own $25,000 bet before it even got off the ground, and I am surprised no-one took him up on it. The reason for this may be that he quickly changed the content of his $25,000 bet page to feature a $1 million challenge to anyone who could “come up with” one or more of the following:
proof of deceit on behalf of Seftel;
proof that the actual medical records “didn't reflect reality”;
proof that “some other thing” caused the outcome;
any other “hard evidence that caused the outcome.”
Kirsch knows this is an impossible bet, because in the absence of an admission by Seftel, it’s hard to prove deceit. The reality may be he had no ill intention at all but, like Kirsch, has a poor grasp of science.
As for proof the medical records showed something other than what Seftel claimed, that would require illegal access to confidential records.
Nice try, Steve.
As far as identifying “some other thing”, identification of the responsible variable/s would require both access to the unobtainable records and interviewing of all Seftel’s patients who, as Kirsch has already stated, are conveniently uncontactable.
At any rate, none of these things are necessary. No mater how Kirsch tries to frame it, the Seftel experience is nothing more than anecdotal observation.
The reason we have randomized, blinded, clinical trials is to determine whether promising anecdotes hold up when subjected to the bright light of tightly controlled scrutiny.
The evidence clearly shows that Seftel story does not hold up when tested in double-blind or even single-blind conditions.
As for Kirsch’s original $25,000 challenge, nowhere has he stipulated that it had a time limit or that the offer has been rescinded. In fact, in his recent article he still maintains there is enough evidence to justify fluvoxamine’s approval as a ‘COVID’ treatment.
Therefore, his offer is still valid.
While he did not stipulate an end date for someone to meet his challenge, Kirsch did claim that there already was enough evidence as of March 21, 2021, and invited responses to his challenge based upon that premise. I have shown that Kirsch himself failed to meet his own stipulated requirements at the time he issued his challenge.
Steve Kirsch owes me US $25,000. I will be writing to him as soon as I post this article to arrange collection of the money.
I’ll keep readers posted.









Just left this comment on his Substack:
"I've been reading Anthony Colpo's work for close to fifteen years, starting with The Great Cholesterol Con. He is one of the most impartial, honest and discerning independent researchers you can find. There are few people's whose work I trust as much as his, or whose writings would cause me to rethink my own premises and positions as much as his on matters of health and nutrition.
"Your refusal to engage with his critique very much calls into question your good faith, particularly given the conflicts of interest he called out in some of the people you are citing. If there is no response to Colpo on this, I will be unsubscribing to your Substack."
Thanks for the mention Anthony. I am not a fan of Big Pharma snake oil packages being sold for self-administration in "Medical Emergency Kits".
https://geoffpain.substack.com/p/deaths-and-birth-defects-from-the