Steve Kirsch Replies to My Challenge
The disingenuous tech millionaire-turned-fluvoxamine shill is now changing the goalposts to avoid paying the $25,000 he owes me
Yesterday, I posted a response to a $25,000 challenge regarding fluvoxamine issued by Steve Kirsch in 2021.
Kirsch harbors an intense obsession with fluvoxamine, a toxic SSRI which he maintains is a “fast, easy, safe, simple, low cost treatment for COVID that has worked 100% of the time to prevent hospitalization.”
I’ve explained here, here and here why fluvoxamine is not an effective treatment for ‘COVID.’
I’ve explained here and here why this drug can hardly be described as “safe.”
Fluvoxamine, however, appears to be the hill Kirsch is willing to die upon.
In April 2020, he created the COVID-19 Early Treatment Fund (CETF), whose express purpose was to find existing drugs that could be “repurposed” as ‘COVID’ treatments. The project attracted plenty of attention from research universities, media praise, millions in donations from Silicon Valley’s elite, and a scientific advisory board staffed with medical all-stars. It was managed by Rockefeller Philanthropy Advisors.
Just over a year later, it all collapsed in a pile. While Kirsch's criticism of the toxic COVID ‘vaccines’ clearly rankled the orthodoxy, it was his obsession with fluvoxamine that ultimately proved to be the CETF’s downfall, according to Doug Richman, distinguished professor of pathology and medicine at the University of California San Diego and a former member of the CETF advisory board. Kirsch was increasingly vocal in promoting the drug as a solution to the pandemic, while the rest of the board were adamant it required more testing. The board’s rising discontent culminated in a wave of abdications in May 2021.
Richman told MIT Technology Review: “[Kirsch] considers himself an expert in something that he doesn’t have training or experience in, and he’s not following scientific methods to assess data.”
Kirsch’s scientific ineptitude was on full display when he issued the following bold challenge on March 21, 2021:
$25,000 reward if you can show we need more data re: Fluvoxamine for COVID-19
Kirsch, who flippantly issues challenges like the army issues boots, was convinced there was already enough scientific data to justify an FDA approval for the use of fluvoxamine in treating ‘COVID.’
So much so, that he offered US $25,000 “to the first person who can provide evidence that shows that the combined evidence from studies published in peer reviewed journals don't already meet that bar.”
His criteria were clear.
"Traditionally," he wrote, "a phase 2 trial plus a phase 3 trial, both with statistical significance, is accepted as evidence. That's a p-value of .0025 or lower. NIH Francis Collins said an effect size of 20% or more would be significant."
He continued:
"For both published randomized studies for fluvoxamine, the effect size was 100%."
The two studies he is referring to were Lenze et al 2020 and Seftel and Boulware 2021.
And this is where Kirsch loses the bet.
Seftel and Boulware 2021 was not a clinical trial, and it was not randomized. It was an anecdotal report of a so-called ‘COVID’ outbreak at a Berkeley racetrack in which Seftel deployed, under totally uncontrolled conditions, fluvoxamine.
The authors (and Kirsch) absurdly describe the study as “quasi-randomized.” Whatever the heck they’re trying to infer, it was not a randomized study. The paper clearly states the patients themselves were allowed to choose whether or not they took fluvoxamine:
Which means, on March 21, 2021, Steve Kirsch did not have evidence that “already meets the bar” for an FDA approval of fluvoxamine as a ‘COVID’ treatment.
He had one (1) randomized trial by Lenze et al, which the authors themselves admitted was exploratory and not a demonstration of efficacy, and an anecdotal report about a ‘COVID’ outbreak in Berkeley.
Kirsch himself failed to meet his own stipulated requirements.
Furthermore, there were no other randomized clinical trials that had been peer-reviewed and published at the time meeting these requirements.
I explained all this in detail yesterday. Within minutes of posting my response to Kirsch’s challenge, I contacted him via his website with the URL, and asked him to contact me so we could sort out the payment.
As explained yesterday, I had previously tried to reach out to Kirsch via his Substack regarding fluvoxamine, providing links to my articles explaining why his recommendation of the SSRI was both erroneous and dangerous.
Those attempts were ignored.
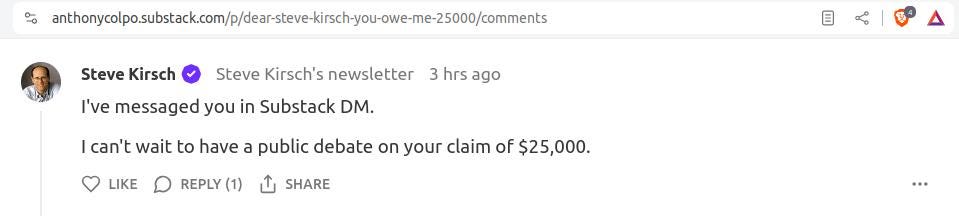
A few hours ago, Kirsch finally stopped ignoring me. Perhaps due to pressure from readers, he left the following comment underneath my response to his challenge:
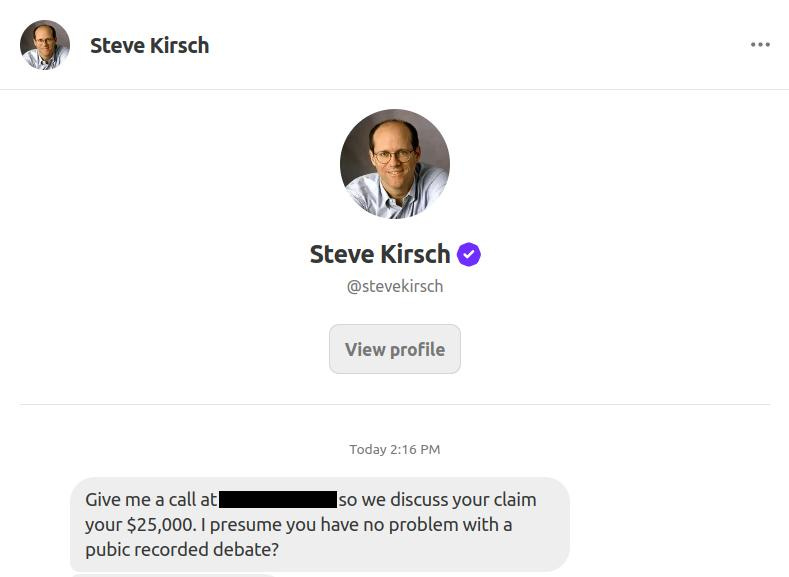
He also sent the following DM.
And so now we see, in all his shape-shifting glory, the real Steve Kirsch.
Nowhere did his $25,000 challenge mention anything about a “debate.”
In that challenge, Kirsch specifically requested “hard evidence” that the two studies he cited “don't already meet that bar.”
I have provided the hard evidence: One of the studies he cited was not a clinical trial and was not randomized.
There were no other published, peer-reviewed randomized clinical trials on the topic when he made his challenge.
There is nothing to debate. Steve Kirsch lost his own challenge and owes me US $25,000.
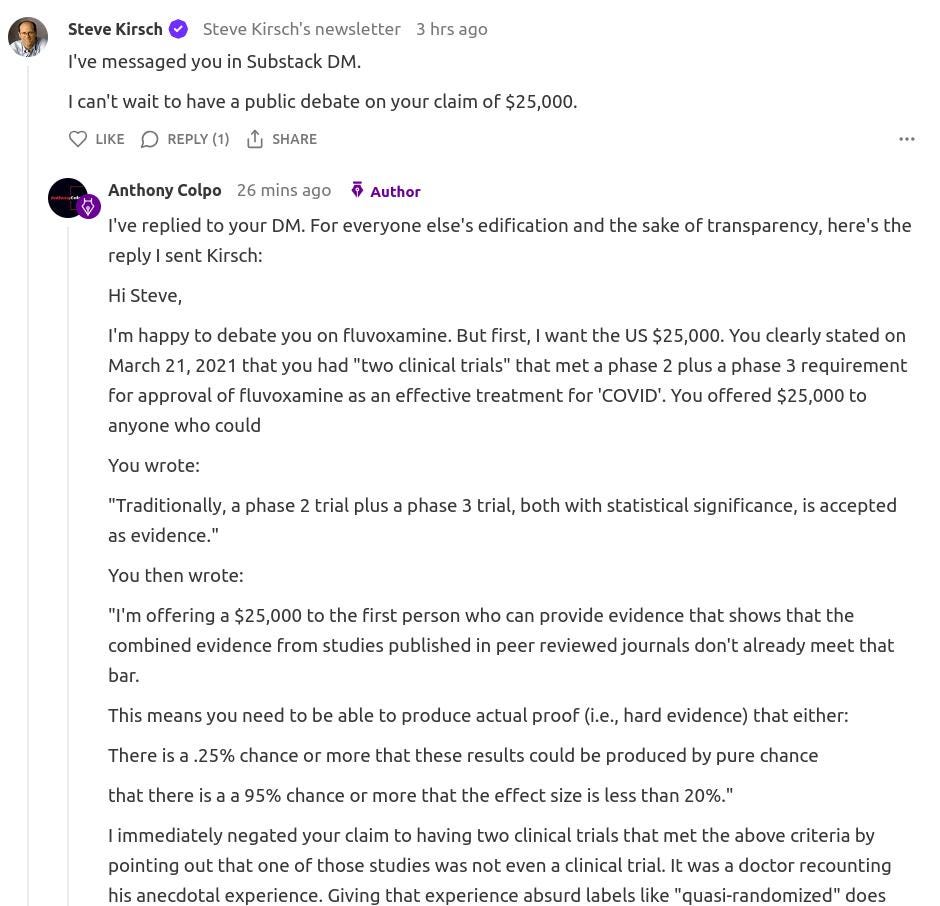
Here is my reply to Kirsch’s comment, which was also sent to him via DM:
Stop trying to wiggle your way out of paying the money you owe me, by now adding a new condition of a “debate” for the money. To be judged by who? You?! One of your unscientific reader polls?!
I’ve already met the conditions you stipulated on March 21, 2021, so it’s time to pay up.
After you’ve paid, then I’ll be happy to debate you on fluvoxamine. Heck, I’ll debate you on Dodge versus Ford, the price of tomatoes, ricotta versus cream in cannoli, whatever.
But first, stop trying to squirm your way out of paying the money that is rightfully owed to me, Kirsch.
Be a man, and live up to your word.










I have immediately left his Substacks, and will follow the struggle. I am not holding my breath on you ever seeing the money.
How a man responds to being called out for falsehood shows his true character. Let's see what happens...