More Problems With the Pfizer-BioNTech COVID-19 Vaccine Trial Data
Back in January, I wrote about some of the inherent problems with the new Pfizer-BioNtech mRNA COVID-19 'vaccine' code-named BNT162b2, and sold under the brand name Comirnaty. To quickly recap:
-The drug is not really a vaccine but a gene-modifying therapy based on long-problematic mRNA technology (this also true of the Moderna drug).
-It's a drug brought to market by a company that is indisputably a corporate criminal, having racked up billions of dollars of fines in criminal convictions, civil penalties and jury awards.
-To approve Cominarty under dubious "emergency use" conditions, regulators used only the first two months' data from a trial that is not scheduled to finish until January 2023!
-The patient information for that clinical trial evinces an unusually healthy baseline patient sample, making the results of questionable relevance to "real world" conditions. At baseline, only 1.5% of the trial participants had suffered a heart attack or heart failure and only 1% had suffered a stroke. The incidence of dementia was so low it was listed as 0%, while a piddling 0.7% had kidney disease (the prevalence of chronic kidney disease in the US is around 14%).
Pregnant or nursing women, people with immune deficiencies or psychiatric issues were excluded from the study. The study protocol was amended to allow participants as young as 12, but the main NEJM paper only discusses data for trial participants 16 years or older.
-Even among this highly-cleansed participant sample, Cominarty still showed a far higher rate of adverse effects than placebo.
-Among the nearly 40,000 subjects included in the NEJM analysis, hardly anyone caught COVID-19. In the vaccine group, eight were reportedly diagnosed with COVID-19 during the first two months, compared to 162 placebo subjects. This is where the "95% effective" figure you see splashed all over the media comes from. But in absolute terms, the COVID incidence figures are 0.04% and 0.88%, respectively. Not even a full percent in either group. What this trial really seems to establish is that COVID-19 is an overblown farce and that the official case numbers have been inflated beyond all get out.
And as I noted in my January article, the ultimate point of the COVID vaccine escapade is to stop the "deadly" COVID-19 virus. But there were no COVID-related deaths in either group. Two Cominarty recipients died (one from arteriosclerosis, one from cardiac arrest), as did four placebo recipients (one from hemorrhagic stroke, another from myocardial infarction and two from "unknown causes").
To claim from this dubious trial, which showed no reduction in COVID deaths and a far higher rate of side effects in the drug recipients, that Cominarty is Safe and Effective!™ is to engage in unabashed charlatanism.
More Problems With the Pfizer-BioNTech Data
It turns out there was a key anomaly I missed when poring over the Cominarty data.
On 4 January 2021, Peter Doshi, associate editor of BMJ, authored a critical opinion piece titled "Pfizer and Moderna’s '95% effective' vaccines—we need more details and the raw data."
Doshi raised a number of concerns, but the one I want to focus on today is the glaring anomaly in the number of people who were ejected from the Pfizer-BioNTech Cominarty trial for no specified reason.
On 10 December 2020, the FDA's Vaccines and Related Biological Products Advisory Committee met to discuss the Pfizer-BioNTech COVID-19 Vaccine. The briefing document is available here, and it contains some interesting figures not included in the main paper published in NEJM.
Scroll down to page 18 of the document, and you'll find a table with exclusion and dropout information (I've reproduced the relevant section below). In any large clinical trial, there will inevitably be participants who drop out due to side effects, because their interest has waned, because they have moved to another city/state/country, because they have developed concerns about being used as a "human guinea pig," and so on.
A number of participants will also inevitably be removed from the study by researchers due to protocol violations, such as missing scheduled clinical visits or not taking or receiving the full treatment as instructed.
This is normal and to be expected.
What is not acceptable is when researchers exploit this phenomenon in order to skew the results in favour of their sponsor's/employer's drug. And make no mistake, this does happen.
Take the notorious Study 329, one of GlaxoSmithKline's numerous misdeeds that, thanks to a whistleblower, came to the attention of New York Attorney-General Eliot Spitzer in 2003. The GSK-sponsored study involved the company's antidepressant Paxil (paroxetine), which like all SSRIs has established solid form for increasing suicidal behaviour. Not a very becoming effect for a drug that is supposed to help depressed people feel better, and one the drug companies - and their friends in the major regulatory agencies - have gone to great lengths to downplay.
Study 329 was led by Brown University's Martin Keller, a Big Pharma darling who received over a million dollars from drug companies just for the 1997-1998 tax periods[1]. Keller and his team brazenly omitted instances of suicidal behaviour in those taking the highly problematic SSRI antidepressant Paxil (paroxetine).
After two weeks on Paxil, a 14-year-old male participant punched some glass-covered pictures and cut himself badly. He was taken to the emergency room of a Fall River Hospital, but because he seemed acutely suicidal he was then transferred to Bradley Hospital, a psychiatric hospital for children and teens in East Providence, Rhode Island. After initial refusals by obstinate Study 329 researchers, his double-blind status in the trial was eventually broken after the director of research at Bradley fired off an angry memo to Keller demanding to know which group the boy was in so that attending doctors could proceed accordingly.
Despite the fact the boy was clearly suicidal and required hospitalization, he was not included among the listed patients suffering serious side effects in the published 2001 paper[2].
Another case uncovered by Spitzer’s office was a fifteen-year-old girl who entered the study in June 1995. In a memo to the institutional review board dated October 30, 1995, Keller wrote the teenager "was hospitalized on [September 15, 1995] due to becoming very combative with her mother and threatening suicide." But instead of coding her behaviour as an adverse effect, Keller said in his memo she was “terminated from the study for non-compliance.”
Another teenage girl was withdrawn from the study after deliberately overdosing on eighty-two Tylenol tablets. Again, instead of being listed among the subjects suffering adverse effects, she was described as having been ejected from the study for being "non-compliant"!
Just like Pfizer and GSK, Eli Lilly is a serial corporate felon. Having lost the patent protection for its blockbuster Prozac in 2001, the company was eager for a new patentable antidepressant. The company's solution was Cymbalta (duloxetine) which, like its predecessor, promptly established a habit of increasing suicide risk. Lilly's solution to this inconvenient development was to to simply ignore as many suicides as possible.
In one of Eli Lilly’s studies, Traci Johnson, a healthy 19-year-old student who had taken duloxetine in order to help pay her college tuition, hanged herself. Her body was discovered on 7 February 2004, hanging by a scarf from a shower rod in an Indianapolis laboratory run by Eli Lilly. It turned out there was no record in FDA files of Johnson and at least four other volunteers known to have committed suicide, and Lilly admitted it never made public at least two of those deaths.
Pfizer itself has engaged in this sort of chicanery to protect the image of its popular antidepressant Zoloft (sertraline). Of sixteen sertraline trials submitted to the now-defunct ClinicalStudyResults.org by Pfizer from the early 2000s, only 7 were published – and only 2 of those 7 studies reported on adverse events. The total number of "suicidal ideation, attempts, injury" reported in those two trials was a big fat zero. However, the summaries for the seven published trials retrieved from ClinicalStudyResults.org in fact showed 5 instances of suicidal behaviour, and the summaries for the 9 unpublished studies showed a further 10.
The disgraceful reality is drug companies can and do manipulate study results in order to hide inconvenient findings and to make their lucrative drugs appear safer and more effective than what they really are.
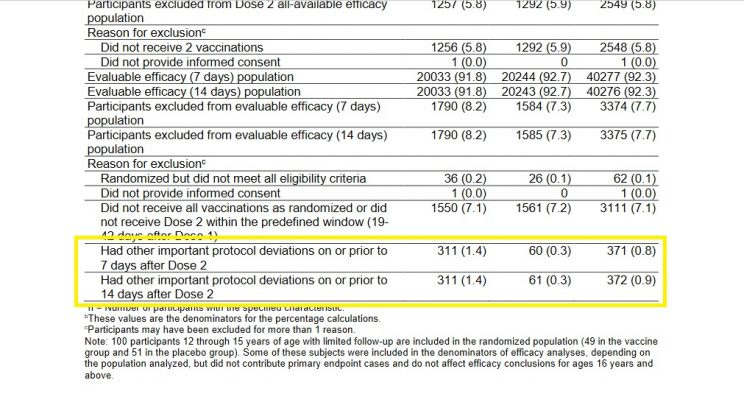
Which makes the exclusion data for the Pfizer-BioNTech trial all the more concerning. Below is a screenshot of the exclusion figures for the study:
The figures in the left-hand column are for Cominarty, those in the middle are for placebo, and those to the right are the combined totals. You'll note that a similar number of subjects were excluded from the study for not receiving all their vaccination/placebo shots within the required time frame - as one would expect in a randomized study.
But take a look at the figures for those excluded due to "other important protocol deviations," which I've highlighted. No specific reasons for exclusion are provided for the folks in this vague category, which makes it a great place for researchers to pull a Study 329-style swifty. Cominarty recipients who experienced negative outcomes could easily have been "excluded" from the study and slotted into this nebulous category, hence favourably skewing the final efficacy and safety analyses.
Lo and behold, we see 311 Cominarty subjects excluded from the study for unnamed "other important protocol deviations," but only 61 in the placebo group. No explanation, of course, is provided for this striking difference.
When Doshi pointed out this highly suspicious anomaly, his critics countered that the mysteriously withdrawn subjects comprised "less than 1% of the study population and can therefore hardly have influenced the study outcome."
Rubbish.
These critics are conveniently forgetting that the total number of people diagnosed with COVID-19 in the study was also less than 1% of the total study population.
In fact, the 250-person difference between the drug and placebo groups dwarfed the total number of COVID cases recorded in the study. In a study with only 170 total COVID-19 diagnoses, and only 6 deaths, a 250-person difference in exclusions for no specific reason could have had a huge impact on the study's results.
Did Pfizer inappropriately exclude a significant number of positive COVID-19 cases, serious adverse effects and/or deaths among Cominarty subjects using the "other important protocol deviations" category?
We don't know, but given the company's long history of criminal dishonesty it's a possibility we simply cannot rule out.
The possibility of data-tampering is heightened by the fact that three Pfizer employees were tasked with the job of "reviewing all potential COVID-19 illness events."
Come on...
Even if these Pfizer employees really were blinded (a generous assumption given the company's nefarious pedigree), it wouldn't be hard for them to nonetheless exclude subjects experiencing adverse events suggesting receipt of the Comirnaty drug (e.g., multi-inflammatory events). Unblinding would also have been facilitated by the fact that up to four times as many Comirnaty recipients received anti-inflammatory and pain medications after their shots. Given the vaccine would be fully expected to produce a higher incidence of soreness and pain than placebo, this information combined with the adverse event data could have helped the researchers correctly guess which group serious adverse events were coming from. A sufficient number of these events could then have been reassigned to the nebulous "other important protocol deviations" category, realigning the results in favour of Cominarty.
Offering up the full exclusion data for independent review would be one way of finding out what is really going on here, but Pfizer isn't playing along. The data-sharing section in its trial protocol says "Pfizer will make available data from these trials 24 months after study completion."
Given that the study completion date is 27 January 2023, this means the data will not be released until 2025 - almost four years away! And even then, Pfizer will be very careful about who it releases the data to. As its data sharing section further states:
"Data requests are considered from qualified researchers with the appropriate competencies to perform the proposed analyses. Research teams must include a biostatistician."
Pfizer itself will decide who has the "appropriate competencies" to scour through its potentially embarrassing data. Only research "teams" with a presumably qualified biostatistician will be allowed to access the data, a stipulation that conveniently excludes capable but solo independent researchers (the type most likely to be free of industry bias) from casting their critical eyes over the data.
And so we have a glaring anomaly in the Cominarty trial data that demands explanation, but no such explanation is forthcoming. All Pfizer and its apologists have to offer us are their time-honoured tactics of evasion and disingenuous rationalizations.
Conclusion
Governments, drug companies and the mainstream media continue their unrelenting campaign to convince us the new COVID-19 vaccines are Safe and Effective!™ However, nothing they say changes the fact these vaccines are not really vaccines, have been poorly studied and rushed to market, and are produced by a bunch of corporate felons with a history of manipulating and falsifying study results.
If you are experiencing suicidal thoughts, please reach out for help. A list of suicide hotline numbers for various countries around the world can be found at the following link:
http://www.suicide.org/hotlines/international-suicide-hotlines.html
References
1. Bass A. Side Effects: A Prosecutor, a Whistleblower, and a Bestselling Antidepressant on Trial. Algonquin Books, Chapel Hill, NC, 2008 (Kindle Edition): 111-112.
2. Bass A. Side Effects: A Prosecutor, a Whistleblower, and a Bestselling Antidepressant on Trial. Algonquin Books, Chapel Hill, NC, 2008 (Kindle Edition): 192-193.
The Mandatory “I Ain’t Your Mama, So Think For Yourself and Take Responsibility for Your Own Actions” Disclaimer: All content on this substack is provided for information and education purposes only. Individuals wishing to make changes to their dietary, lifestyle, exercise or medication regimens should do so in conjunction with a competent, knowledgeable and empathetic medical professional. Anyone who chooses to apply the information on this substack does so of their own volition and their own risk. The author/s accept no responsibility or liability whatsoever for any harm, real or imagined, from the use or dissemination of information contained on this substack. If these conditions are not agreeable to the reader, he/she is advised to leave this substack immediately.